Media Release: Nelson, New Zealand – 10 July 2024 – Kimer Med $10M closer to…
Rebuttal to OpenPhilanthropy.org’s list of objections to DRACO
Some time ago, the folks at openphilanthropy.org posted a long article with their objections against DRACO: https://www.openphilanthropy.org/informal-writeup-dracos-potential-antiviral-treatment
No one has yet undertaken a rebuttal, so I thought the time is right. I can’t say I’m thrilled about the idea of posting and discussing a link with so much misinformation in it, but given the likelihood of it coming up in any serious Google search or due diligence investigation, it’s better to just address it head-on.
Chris Somerville spent 2 hours reading about DRACOs and then discussed for about an hour with Holden. Chris, who is not a virologist, emailed a friend with knowledge of virology about DRACOs for a quick opinion.
In other words, the authors aren’t experts, and only spent a few hours thinking about things. This gets more obvious as time goes on.
Complicated delivery: For DRACOs to work as a therapy, there needs to be an efficient method for therapeutically delivering proteins inside of cells in intact animals. Protein motifs (PTDs) that can induce uptake by cultured cells were used in the Rider paper and have been widely used experimentally. By contrast, delivery of therapeutic proteins into cells in live animals is relatively poorly described in the literature.
Protein transduction tags (PTDs) were demonstrated in Rider’s paper as working effectively in live mice. The paper describes successful and safe treatment of mice against Influenza H1N1 using DRACO, including tags to deliver the protein into cells.
In addition, earlier research showed the effectiveness of transduction tags in live mice. For example:
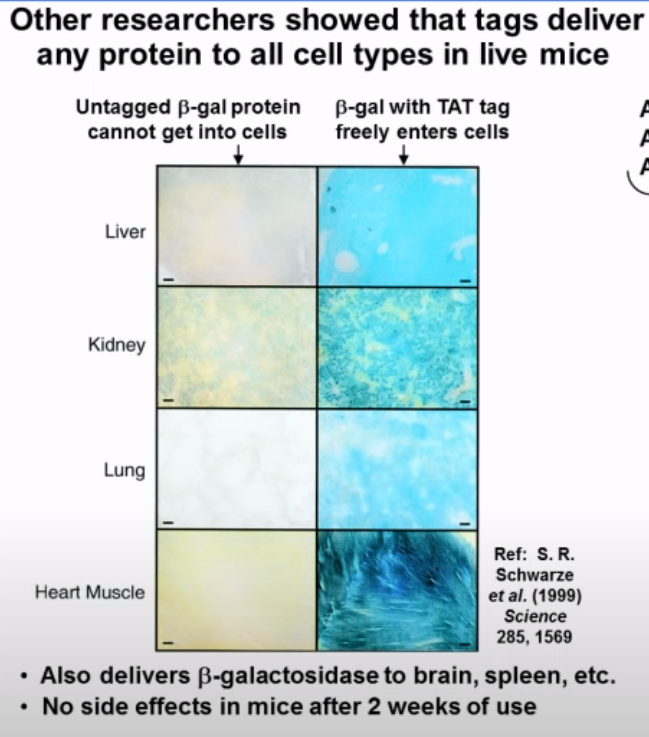
The graphic above shows how a transduction tag delivered a protein into a wide range of tissue types in mice, including the brain (so it passes the blood-brain barrier), and that there were no side effects in these mice after 2 weeks of use.
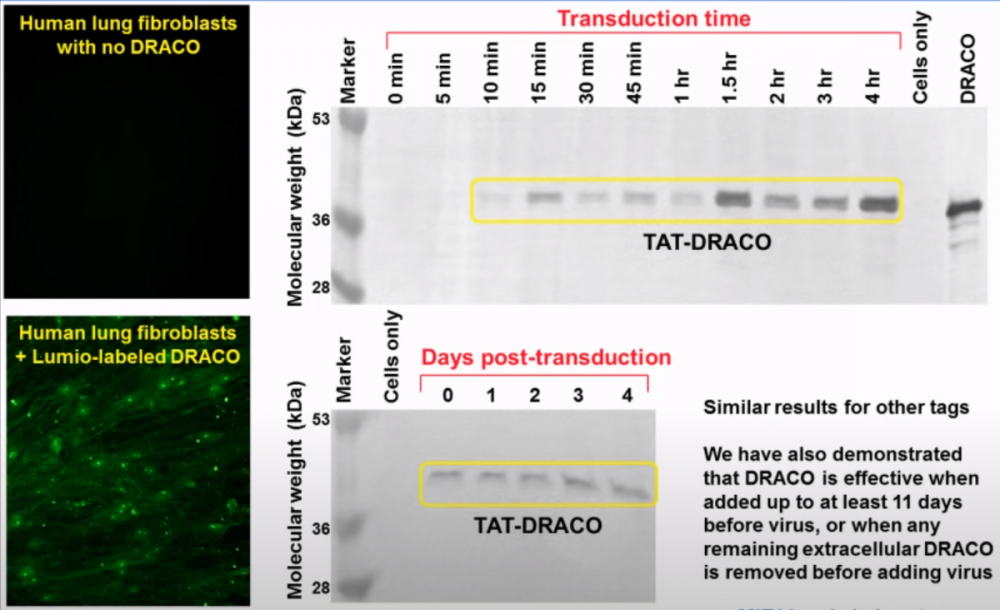
On the left in the graphic above, a fluorescence study visually shows the effectiveness of transduction tags at carrying DRACO into human lung fibroblast cells. With the TAT tag, as above, the concentration of the protein in the nucleus appears higher than the cytoplasm, although there is still a significant amount in the cytoplasm.
The diagram also shows how the concentration of DRACO in cells increases over time, first appearing at about the 10 minute mark, reaching a maximum after about 1.5 hours, and lasting for at least 11 days.
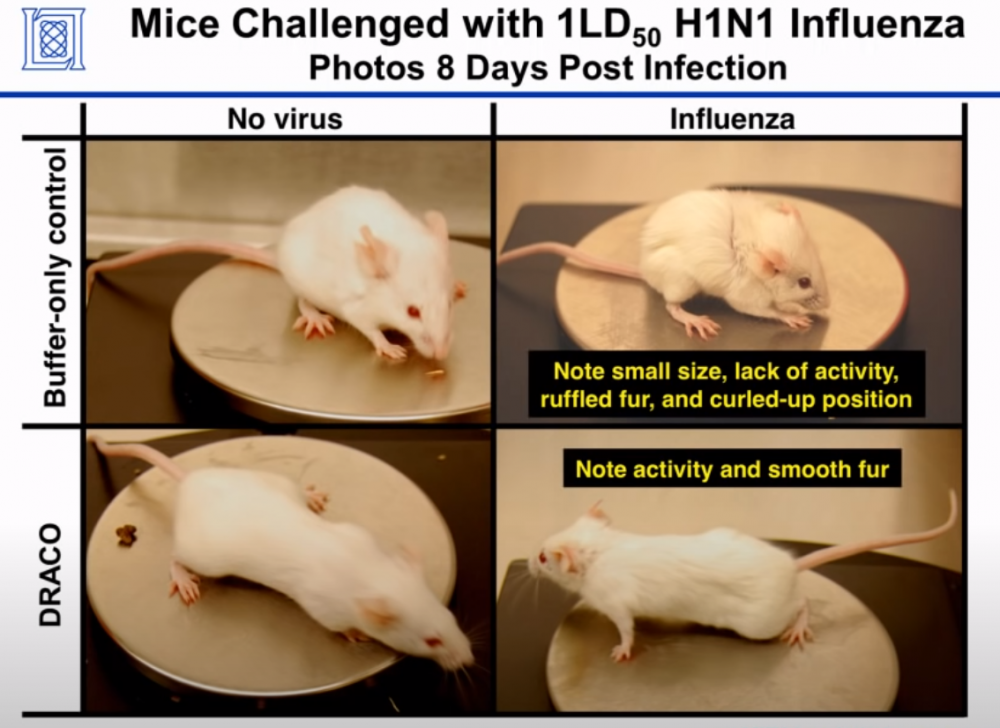
The ultimate test of the effectiveness of transduction tags was the fact that DRACO performed as expected against 15 different types of viruses in 11 different tissue types, and against 3 different viruses in mice (only Influenza was mentioned in the paper; the other two came later). As in the prior studies, the DRACO-treated mice were happy and healthy:
As of 2019, more than 1700 CPPs were known, and there were more than 25 CPP-conjugated drugs were under development.
Is it possible that there are some cells or tissues which transduction tags won’t reach in adequate quantities when DRACO is injected, ingested or inhaled? Sure, it’s possible, although you could say the same thing about any drug and administration method. But to say the mechanism is “complex” and “poorly described” in animals is disingenuous at best. It’s actually a simple, safe and extremely elegant cellular delivery mechanism.
Whatever delivery method there is would likely have to be more complicated than a pill and might require skilled administration, limiting potential for widespread or quick use.
Rider’s paper used two methods of administration: intraperitoneal injection (i.p.) and intranasal (i.n.). I.p. injection in mice is often used simply because it’s easier than other forms of parenteral administration. I.n. basically consists of letting the mouse inhale the substance through its nose (see the short video below). Neither of these are complex in mice.
In humans, we don’t know the best method of administration yet, and in fact it might vary from one virus to another. The equivalent of i.n. in people would just be a nose spray; i.p. would be an intravenous injection or IV drip. In mice, i.n. was effective in treating Influenza, partly because the lungs and airways are the tissues most affected by the virus. The same may be true in humans.
It’s possible that oral administration will be possible in humans, particularly for viruses that affect the gut, but also systemic. Although DRACO is a protein, there are many well-established ways to effectively protect proteins from enzymes and early digestion (enteric coatings, for example).
Even though the most effective method of administration is something we intend to investigate, there’s nothing here that limits the potential for widespread or quick use.
Dangerous: For DRACOs to work, it would be necessary to deliver a lot of protein to cells (because DRACOs have to get into all cells that might be infected). Based on the mouse studies reported by Dr. Rider, it seems possible that a gram or more of DRACO would need to be administered to humans
The doses used in the paper for i.p. were 0.8 to 2.5 mg in 200 ul (4 to 12.5 g/L concentration), or for i.n. 0.5 mg in 50 ul (10 g/L). The conventional rule-of-thumb scaling factor between mouse and human dosing is based on skin surface area. On a mg/kg basis, that works out to dividing by a factor of 12.3. So, a little math: 2.5 mg absolute / 50 g typical mouse = 50 mg/kg. Scaling to humans: 50 / 12.3 = 4.1 mg/kg. Scaling to a 70 kg human: 4.1 * 70 = 287 mg. If it ends up being possible to take it orally and in powder (perhaps lyophilized / freeze-dried) form, that’s about the same as a medium-sized vitamin pill.
In terms of injection amount, assuming the i.p. concentration of 12.5 g/L, a 287 mg dose would be about 23 cc. Although that’s too much fluid for I.M. or simple by-hand venous injection, it’s easily done with an I.V. drip. Note that Rider doesn’t say what the concentration of a saturated solution is; it’s possible that we will be able to make it much more concentrated than he did.
However, there’s an important caveat about the above numbers. Rider also measured the minimum dose at which DRACO was fully effective, and found it to be only 10 nM/L, which is unusually low as conventional drugs go; it’s roughly the same order as human hormones. For example, the concentration of typical human thyroid hormone (T4) is about 100 nM/L. The oral dose of T4 required to maintain homeostasis is on the order of 100 mcg/day. If we crudely apply the same scaling to DRACO, it suggests a dose of only 10 mcg might be enough to achieve the effective in-cell level of 10 nM/L. That’s a factor of some 28 million less than the dose used in the original study.
Dr Rider clearly didn’t (and couldn’t) know what the required dose would have to be before he started his study. My take on his work is that he gave the mice the highest dose he could that he thought wouldn’t be toxic. Importantly, even at the high mg/kg doses he administered to mice, the mice still didn’t show any toxic effects. One of things we plan to test for is exactly what the dose really needs to be in people, but as I’ve explained above, we have good reason to be hopeful that it can be quite low.
Because DRACOs contain a non-human component, it is very likely that they will induce a strong immune response.
On the theory side, DRACOs are mostly made from parts of two existing human proteins. The only non-human component is a very short peptide (roughly 9 to 11 amino acids), which is the transduction tag discussed above. Based on this, while some immunogenic response is possible, as with all drugs, the odds of it being significant are small. It’s certainly much safer and less immunogenic than current approaches that use entire active viruses to deliver payloads inside cells.
On the practical side, the mice that were treated with DRACO in the 2011 study showed no signs of either toxicity or immunogenecity, in spite of the relatively high doses they received.
Viruses sometimes infect large numbers of cells. If DRACOs succeeded in killing all human cells with viruses in them, that could mean killing very large numbers of human cells, potentially posing substantial health risks.
This is a valid concern. One of the ways that VTose® is different from DRACO is that we have several potential solutions. I can’t say too much more at the moment, though, other that this is something we’ve given considerable thought and attention to.
Nature could have evolved a mechanism for triggering apoptosis in cells with dsRNA, but appears not do it naturally.
Yes, nature could have done that — and did:
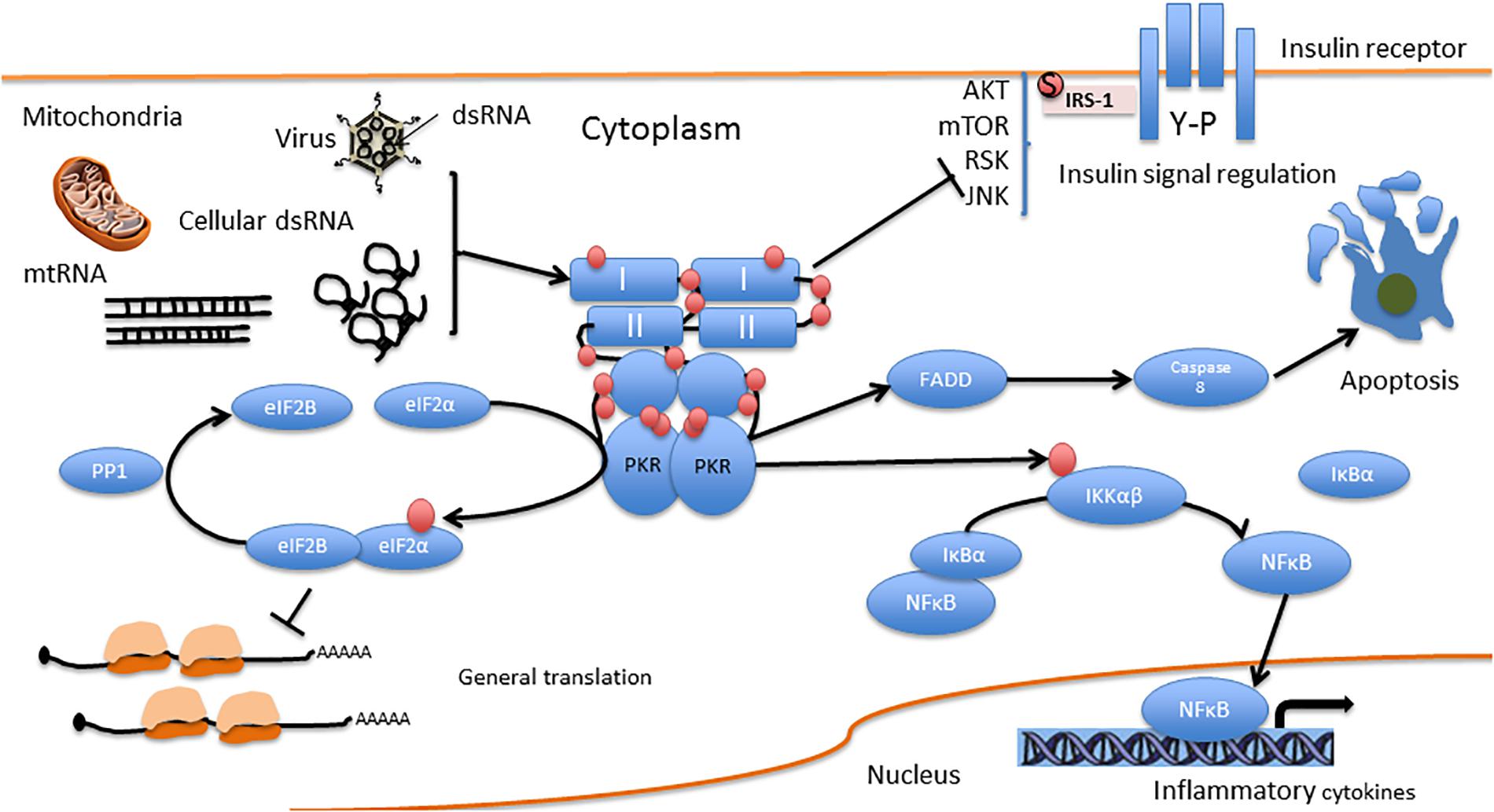
You can see PKR in the middle of the diagram, attached to dsRNA, which triggers FADD, then Caspase 8, which causes apoptosis.
The problem with this pathway is that viruses like to interfere with it. For example, Caspase 8 is a common target:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4515451/
There is a risk that some cells may naturally have enough dsRNA that they could potentially be attacked by DRACO despite being healthy. The proteins used in the DRACO system are most strongly activated by long dsRNA which is intended to make the mechanism specific for viruses. However, short dsRNA of 11-16 nucleotides can bind to the dsRNA binding sites and activate the proteins under some conditions
The full PKR protein is already present in all mammalian cells. Activation of PKR (and therefore DRACO) requires binding of two molecules of to dsRNA, as shown in the diagram above. This requires at least 30 to 50 base pairs of dsRNA; short segments don’t and can’t cause activation. If they did, they would activate PKR in healthy cells, along with all of its downstream effects. Uninfected mammalian cells generally do not produce dsRNA longer than 21 to 23 base pairs.
Harmless viruses in cells could be targeted by DRACOs as well, posing additional risk (i.e., widespread cell death).
I would like to know which viruses the author considers harmless. There are viruses like CMV and EBV that often produce subclinical symptoms, but there’s also clear evidence that they’re harmful. The widespread cell death issue was addressed earlier.
Bacteriophages are viruses that attack bacteria, and may be helpful as a result. As far as we know, DRACO doesn’t affect bacteriophages.
In light of the safety concerns above, it may not make sense to use DRACOs to treat low-stakes conditions like rhinovirus (the common cold).
The safety concerns were debunked above. It’s possible that a low-dose DRACO administered by nose spray may end up being an inexpensive and effective treatment for the common cold. We already know DRACO is effective against Rhinovirus, so that’s an important first step. As with any new drug development, there are, of course, questions to be answered and investigations to be done. However, based on what we know today, we don’t see any show stoppers.
While it might make sense to use a risky treatment against a very deadly virus, DRACOs would not work against HIV because this virus does not rely on dsRNA.
HIV is a Lentivirus, which is a positive-sense single-stranded RNA enveloped retrovirus:
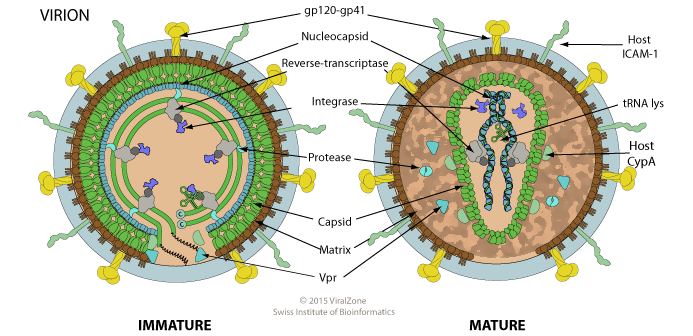
These viruses are similar to Dengue virus, which DRACO was found effective against in Rider’s paper:
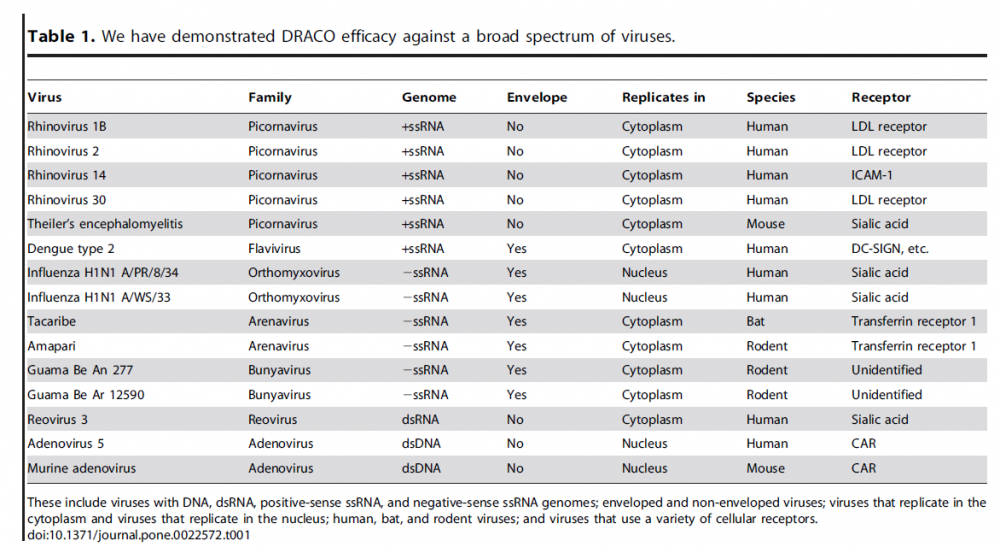
SARS-CoV-2 is also a (+)ssRNA enveloped virus.
Although HIV’s viral genome uses ssRNA, the viral replication process still uses dsRNA — as do all viruses.
One difference is that Dengue is not a retrovirus like HIV, nor were any of the tested viruses, and retroviruses do behave a bit differently in some ways than other viruses. However, since dsRNA is still present while a retrovirus virus is replicating, there’s good reason to believe DRACO will be effective. We do, of course, need to test to be sure.
The Rider paper presented some preliminary mouse trials which indicated significant protection against life-threatening viral infections. However, the trials were quite short and there was no detailed analysis published of effects on the overall health or longevity of the mice. If further experiments were done, the emphasis should probably be on long-term health effects on a variety of animals with and without viral infections
I agree with this point. Rider described his trials with mice as a “proof of concept.” We learned many interesting and useful things as a result, but more work remains, including longer-term studies.
Crowdfunding page cites the “valley of death” as an explanation for why the NIH is not funding work on DRACOs. Chris is skeptical of this explanation. He thinks that antivirals are a very hot area, and that it’s generally easy to get funding and interest around in vitro/cheap-animal trials of things that have worked before. Given above point, Chris thinks it’s a bad sign that there has been so little follow-up research since the Rider paper.
What I’ve heard is that it’s generally easy to get NIH funding for basic research, and for the final take-it-to-market step, but not for the in-between phase that involves clinical trials and the like. But I wasn’t there, so I can’t say for sure what happened. I have done my fair share of US Gov-funded research though, and can say with some authority that they are a fickle beast, and that politics and hidden third parties are often involved. From where I’m sitting the lack of NIH funding simply means someone at NIH decided there was some reason not to fund it. It tells me exactly nothing about what that reason was. What the rest of us should be doing is looking critically at the science in the paper. Surprisingly, the critique on which this thread is based seems to have completely avoided that approach.
Given the points above, Chris’s guess is that there are further reasons and possibly further evidence for the unpromising-ness of DRACOs that we aren’t seeing.
Guesses and possibilities about things imagined aren’t helpful. At this stage of the paper, and as I hope you can see from my rebuttal above, it sure looks to me more like a hit piece, and less like honest journalism.


